During the scientific trials for its COVID-19 vaccine, pharmaceutical large Pfizer seems to have hidden two deaths — together with one in Kansas — which researchers allege would have revealed doubtlessly harmful uncomfortable side effects to the vaccines.
Over the final 12 months and a half, a workforce of researchers — volunteering for The Daily Clout, a non-profit information outlet — together with physicians, a businessman, and a former United States Army Intelligence officer poured by means of hundreds of pages of paperwork regarding the examine and located that Pfizer had did not report the deaths of two girls — one in Kansas and one in Georgia — throughout the trial.
Not solely did they fail to report them, however that they had—in truth—apparently actively lined them up.
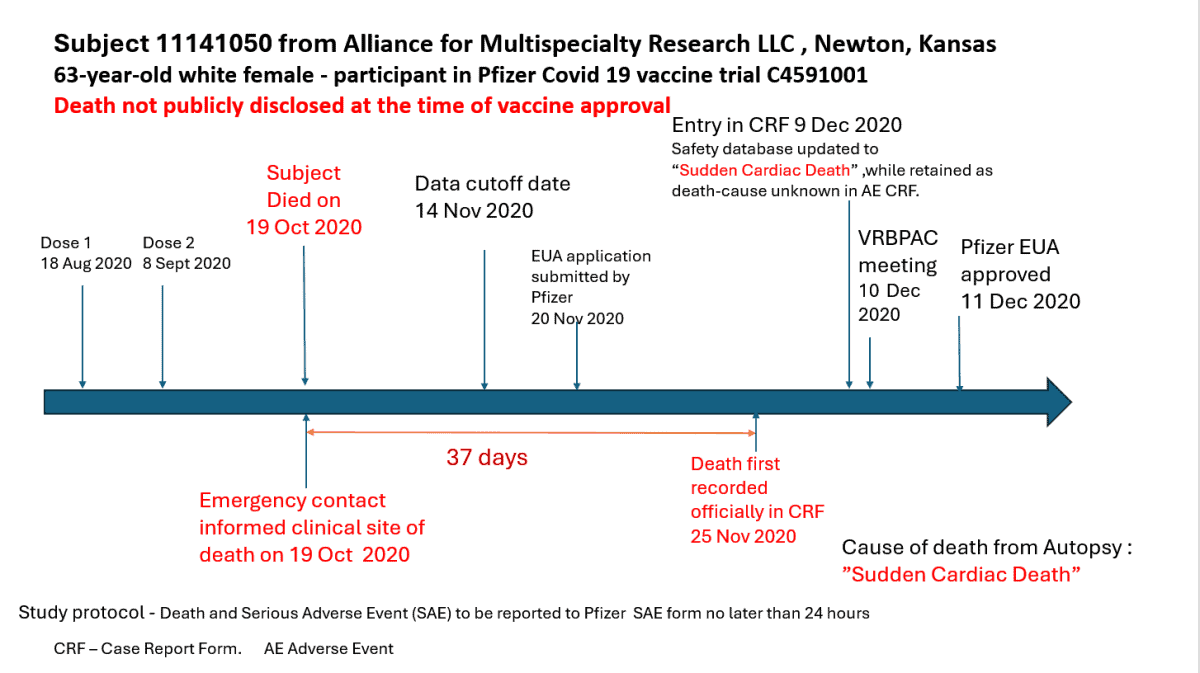
According to Dr. Jeyanthi Kunadhasan, an anesthetist and perioperative doctor in Australia; who was a part of the workforce, the examine protocol required that any “loss of life or severe adversarial impact” needed to be reported inside 24 hours. In the Kansas case that didn’t occur for 37 days.
The Kansas case was a 63-year-old lady who had her first dose of the Pfizer mRNA vaccine on August 18, 2020, and a second dose on September 8, 2020. She died on October 19, 2020, and her emergency contact instantly knowledgeable the scientific web site — Alliance for Multispecialty Research LLC, in Newton, Kansas. Thirty-seven days later, on November 25, 2020 — 11 days after the information reporting cutoff date — the loss of life was lastly recorded in a “case report kind.”
Five days after the emergency use utility was submitted to the Food and Drug Administration by Pfizer.
The participant’s loss of life was not reported within the trial ends in the celebrated New England Journal of Medicine or to the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), which accepted the EUA.
According to a letter Kunadhasan sent to Kansas Attorney General Kris Kobach — who’s suing Pfizer over the vaccine — an post-mortem recorded the trigger as “sudden cardiac loss of life,” and Pfizer physicians dominated her loss of life was not associated to the vaccine, citing “danger elements” for coronary heart illness to incorporate hypertension and weight problems.
However, in line with Kunadhasan, the affected person was hardly overweight however fairly mildly obese, at roughly 5 toes 4 inches tall and 163 kilos.
“To be eligible for inclusion on this scientific trial, members needed to be deemed wholesome based mostly on medical historical past, bodily examination (if required), and the scientific judgment of the investigator. The protocol allowed wholesome members with pre-existing steady illness – outlined as illness not requiring important change in remedy or hospitalization for worsening illness throughout the six weeks earlier than enrolment – to take part within the scientific trial,” Kunadhasan wrote. “This affected person was medicated with two totally different antihypertensives and had encountered scientific trial personnel no less than 3 times with no point out of any worryingly hypertension readings. In truth, I can not discover any blood strain studying in her publicly obtainable case notes. Consequently, I can solely assume the affected person’s hypertension, from which she had suffered since January 1st, 2010, was well-controlled when she was admitted to the trial.
The affected person was 165cm tall and weighed 74.1kg. Hence, her BMI (physique mass index) of 27.2 put her within the obese class, not overweight.
The second hidden loss of life was a 58-year-old lady in Georgia.
She obtained her first dose on August 4, 2020, and a second on August 27, 2020. The lady died in her sleep on November 7, 2020, and her husband instantly knowledgeable the scientific web site. The loss of life was not added to the information for 26 days and first adopted up on December 3, 2020 — once more properly after the Nov. 14 knowledge cutoff date.
The lady had taken a muscle relaxer and valium for persistent again ache previous to going to mattress, however each had been drugs “beforehand utilized by the topic.”
In a letter to Texas Attorney General Ken Paxton, who — like Kobach — is suing Pfizer, Kunadhasan notes that there was no post-mortem — a coroner was merely referred to as to pronounce loss of life — and the trial investigator merely declared there was “no affordable risk that the cardiac arrest was associated to the examine intervention.”
However, with out an post-mortem, how can anybody know?
Why do two deaths matter?
Dr. Chris Flowers, a member of the workforce and retired professor of Radiology who has labored scientific trials for greater than 40 years, stated even these two deaths out of the simply over 44,000 members ought to have been a sign to cease the trial instantly till they might make certain the vaccine was not the explanation for the loss of life. Flowers stated an instance could be the swine flu vaccine trial through which a participant received Guillain Barre Syndrome, and the trial was stopped.
“So for those who put it in context, sure, there’s a small variety of deaths,” Flowers stated. “But any loss of life which may be because of the intervention that you simply’re doing — the remedy you’re giving — is extraordinarily essential, and usually the FDA calls a cease to these scientific trials till additional investigation is completed. And in lots of instances, is form of the loss of life knell of that scientific trial.”
The proven fact that each members died of coronary heart assaults turns into extra essential when different research showed risks of myocarditis and pericarditis — significantly after a second shot and significantly in younger males below 25, however amongst different sufferers as properly.
Moreover, the studies proven to VRBPAC and within the New England Journal of Medicine acknowledged there have been solely six deaths within the trial, 4 within the placebo group and two within the vaccinated group, supposedly exhibiting that the vaccine labored and lowered the danger of loss of life.
However there have been truly 4 in every group.
“Nonetheless, the September 2023 Pfizer-BioNTech knowledge launched by the FDA launched a doc named “125742_S1_M5_5351_c4591001-interim-mth6-narrative-sensitive.pdf,” which included data revealing that Pfizer-BioNTech was, in truth, knowledgeable of two extra deaths within the BNT162b2 arm of the trial properly earlier than the EUA knowledge deadline, and that Pfizer-BioNTech didn’t disclose these deaths to the FDA,” Kunadhasan’s letter to Paxton reads. “If the deaths had been disclosed within the EUA submission, they might have proven that the BNT162b2 mRNA COVID vaccine intervention didn’t scale back deaths.”
The backside line? Kunadhasan believes Pfizer knew the vaccine didn’t work and will trigger coronary heart issues — even earlier than it was accepted.
“There was a cardiovascular sign that we discovered within the knowledge from the deaths,” Kunadhasan stated.
In additional tales, the Sentinel will discover extra issues with the way in which the examine was carried out and its failure to point out actual scientific profit.
Post Views: 7,116