Using a intelligent mixture of light-triggered chemical reactions, a staff of researchers has managed to insert a thread-like molecule right into a ring-shaped molecule in a high-energy association — this “molecular match” is one thing that may’t occur naturally.
What makes this experiment particular is that it created a construction that’s not in thermodynamic equilibrium, which is a uncommon achievement relating to synthetic methods.
An synthetic system like a nanomotor is fashioned when its molecular parts self-assemble and attain a state of thermodynamic equilibrium. However, relating to residing beings, they operate whereas staying away from self-assembly and thermodynamic equilibrium.
Preventing self-assembly and “reproducing such (residing being-like) mechanisms with synthetic methods is a fancy and bold problem that, if met, might allow the creation of latest substances, which may very well be used to develop, for instance, good medicine and energetic supplies,” the researchers stated.
Disrupting the self-assembly of a man-made system
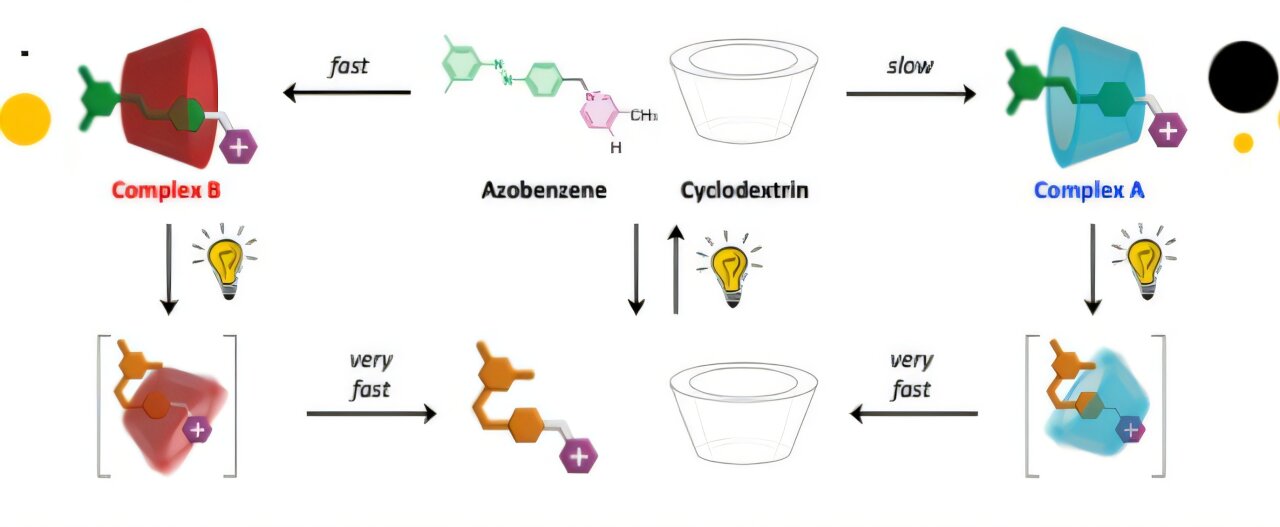
The examine authors used two molecules for his or her experiment; the ring-shaped cyclodextrin and a filiform (thread-like) azobenzene spinoff. The former is manufactured from glucose, it’s used as a drug provider, meals additive, and purification agent. Whereas the latter has the uncanny means to alter form when uncovered to mild.
When each molecules interacted in water they resulted within the formation of two complexes; complicated A and sophisticated B. Out of the 2, A is thermodynamically secure, types slowly, and may exist even within the absence of sunshine.
However, when the 2 molecules interacted within the presence of seen mild, the azobenzene molecule bends, inflicting the complicated to interrupt aside as a result of its new form not suits the cyclodextrin cavity.
This disruption within the self-assembly of the system resulted within the formation of fast-forming however much less secure complicated B. The short-lasting unstable complicated B represents a novel product that may’t be created when a man-made system follows its authentic nature.
“The self-assembly mechanism coupled with a photochemical response makes it doable to harness the vitality of sunshine to build up unstable merchandise,” the examine authors stated.
A secret to creating novel units
When the sunshine is switched off, azobenzene returns to its authentic form, and sophisticated A is fashioned. You gained’t see any signal of the thermodynamically unfavored complicated B.
However, this experiment has demonstrated that it’s doable to create novel synthetic methods by manipulating their self-assembly mechanism.
For occasion, the researchers declare that by utilizing their method and bettering it additional, one can develop new chemical synthesis strategies and units like nanomotors that may work in non-equilibrium situations.
“The simplicity and flexibility of our method, along with the truth that seen mild – i.e., daylight – is a clear and sustainable vitality supply, permit us to foresee developments in varied areas of know-how and medication,” Alberto Credi, one of many examine authors and a professor on the University of Bologna, stated.
The examine is revealed within the journal Chem.




