Sign up for the Starts With a Bang e-newsletter
Travel the universe with Dr. Ethan Siegel as he solutions the largest questions of all
Here on planet Earth, in addition to in most areas within the Universe, every little thing we observe and work together with is made up of atoms. Atoms are available roughly 90 completely different naturally occurring species, the place all atoms of the identical species share comparable bodily and chemical properties, however differ tremendously from one species to a different. Once regarded as indivisible models of matter, we now know that atoms themselves have an inside construction, with a tiny, positively charged, large nucleus consisting of protons and neutrons surrounded by negatively charged, a lot much less large electrons. We’ve measured the bodily sizes of those subatomic constituents exquisitely properly, and one reality stands out: the scale of atoms, at round 10-10 meters apiece, are a lot, a lot bigger than the constituent components that compose them.
Protons and neutrons, which compose the atom’s nucleus, are roughly an element of 100,000 smaller in size, with a typical measurement of solely round 10-15 meters. Electrons are even smaller, and are assumed to be point-like particles within the sense that they exhibit no measurable measurement in any respect, with experiments constraining them to be no bigger than 10-19 meters throughout. Somehow, protons, neutrons, and electrons mix collectively to create atoms, which occupy a lot larger volumes of area than their parts added collectively. It’s a mysterious undeniable fact that atoms, which have to be principally empty area on this regard, are nonetheless impenetrable to 1 one other, resulting in monumental collections of atoms that make up the strong objects we’re conversant in in our macroscopic world.
So how does this occur: that atoms, that are principally empty area, create strong objects that can not be penetrated by different strong objects, that are additionally made from atoms which might be principally empty area? It’s a exceptional reality of existence, however one which requires quantum physics to clarify.

Although human beings are made from cells, at a extra elementary degree, we’re made from atoms. All advised, there are near ~10^28 atoms in a human physique, principally hydrogen by quantity however principally oxygen and carbon by mass.
If you need an instance of a strong object, look no additional than your self: a human being. Although you’re a set of atoms — roughly 1028 of them in case you’re a full-grown grownup — you’re nonetheless a strong object: you’ve a definitive quantity and form, and solely by means of puncturing or severing the bonds that maintain your very atoms collectively can one other object that’s additionally made from atoms “move by means of” you. Right now, there’s likelihood that you simply’re in touch with different strong objects: clothes, footwear, the ground, a chair, and many others. Somehow, despite the fact that they’re principally empty area, and despite the fact that they’re all made from the identical primary constituents, atoms which might be part of you stay part of you, and atoms which might be part of the opposite objects you’re in touch with stay part of these objects.
It’s as if one thing is compelling the atoms that compose you to stay part of you, whereas refusing to include and admit atoms which might be part of another object. Even in case you had been to press your thumb as arduous as you could possibly into the seat of the chair that you simply’re sitting on, the atoms of the chair will stay part of the chair and the atoms of your thumb will stay part of your thumb; these two collections of atoms won’t ever move by means of each other the best way that X-rays may move by means of your physique’s atoms. Ever since we realized what atoms are made from, and decided that the scale of the atom is far, a lot bigger than the bodily measurement of its constituents, we’ve been compelled to marvel why that is so.
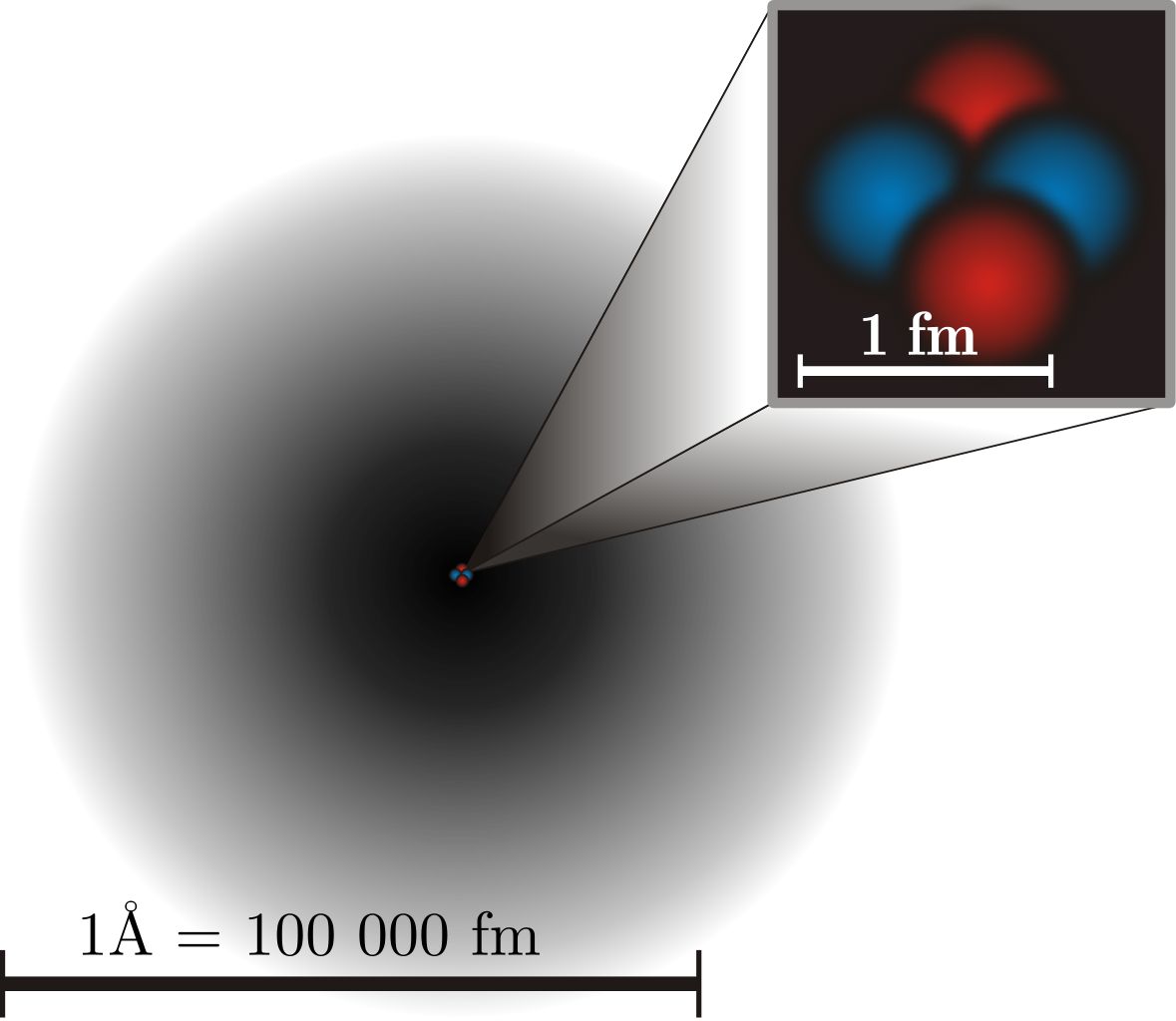
Although, by quantity, an atom is generally empty area, dominated by the electron cloud, the dense atomic nucleus, answerable for just one half in 10^15 of an atom’s quantity, comprises ~99.95% of an atom’s mass. Reactions between inside parts of a nucleus could be extra exact and happen on shorter timescales, in addition to at completely different energies, than transitions restricted to an atom’s electrons.
An atom, in any case, is a quantum mechanical object with a large, positively charged nucleus that’s surrounded by a really low mass, negatively charged cloud of electrons round it. While early photos of the atom displayed an analogous construction to our Solar System, we now comprehend it’s way more correct to view the electrons as cloud-like in nature reasonably than as point-like. There’s a tremendously elementary purpose for this: the very fact of quantum uncertainty, and that each one quanta, together with electrons, exhibit wave-like habits. In specific, each particle of matter could be described as a wave, with a attribute wavelength scale decided by its momentum.
It’s this view of the electron, surrounding the nucleus with solely a discrete set of vitality ranges out there to it, that we usually attraction to after we speak in regards to the measurement of atoms. Atoms which might be extra large — i.e., which have bigger numbers of protons and neutrons of their nucleus — have extra electrons in orbit round them, because the variety of electrons in a impartial atom all the time matches the variety of protons within the nucleus. Because of the character of atom-based matter, and the way electrons fill atomic shells earlier than transferring on to occupy the following out there vitality ranges, atoms with larger numbers of electrons, and with larger numbers of crammed atomic shells, are typically bigger in measurement than atoms with fewer electrons and fewer crammed shells.

Although, at a elementary degree, the Universe is made up of point-like quantum particles, they assemble collectively to create objects of finite sizes and lots more and plenty, occupying particular quantities of quantity. This artist’s illustration exhibits a number of electrons orbiting an atomic nucleus, the place the electron is a elementary particle, however the nucleus could be damaged up into nonetheless smaller, extra elementary constituents. Whether there are constructions on scales smaller than the presently recognized subatomic particles stays to be found.
It’s these properties of atoms and of electrons that allow the chemistry that’s the hallmark of Earth’s complexity. Atoms bind collectively based mostly on the interactions between the electrons round their atomic nuclei, forming molecules, ions, and all types of intricate constructions. In a large number of circumstances, these can construct as much as kind macroscopic constructions, the place many of those constructions tackle strong, definitive, unchanging shapes. At their core, they might merely be massive collections of atoms all sure collectively, nevertheless it’s the kind of atom, the preparations of these atoms, and the bonds they kind with each other that each one add as much as decide the properties of the macroscopic object we wind up contemplating.
However, for any molecule, regardless of how massive, the story of its constituent electrons winds up being similar to the story of even a single atom. Electrons fill the lowest-energy shells inside it, with essentially the most loosely-held electrons primarily figuring out the bodily and chemical properties of the general object/molecule in query. Some objects decide up electrons from their environment simply; different objects have their electrons simply stripped away; some objects bind along with different objects very simply to kind even bigger, extra complicated sure states. Yet, at their cores, it’s the electrons that do or don’t fill the vitality ranges that in the end decide the properties of the article most importantly of all.

The atomic orbitals of their floor state (high left), together with the next-lowest vitality states as you progress rightward after which down. These elementary configurations govern how atoms behave and exert interatomic forces.
And but, if you carry two completely different objects collectively — say, your physique and the chair you’re sitting in — the atoms that compose every object stay a part of every particular person object beneath most circumstances. The two objects by no means move by means of each other, despite the fact that they’re principally empty area. You may suppose that there are two causes for this:
- quantum uncertainty, which causes electrons to unfold out over a bigger quantity of area,
- and electrostatic repulsion, which allows all like-charged particles, like negatively charged electrons, to repel one another.
Put these two elements collectively, and also you may assume that you simply’ve bought a recipe for atoms to create strong, impartial objects.
The quantum uncertainty inherent to electrons makes them occupy a big (in comparison with the scale of an atom’s nucleus) quantity, and if you bind atoms collectively, these electron clouds then occupy even larger areas of area. Similarly, as a result of the electrons occupy the outermost components of those atoms and molecules, the act of bringing distinct objects shut collectively means their electrons get very shut to 1 one other. Since like expenses repel, and all electrons have the identical unfavorable cost inherent to them, this creates a narrative for a way objects composed of atoms might come to be strong on macroscopic scales.

From macroscopic scales all the way down to subatomic ones, the sizes of the elemental particles play solely a small function in figuring out the sizes of composite constructions. Whether the constructing blocks are really elementary and/or point-like particles remains to be not recognized, however we do perceive the Universe from massive, cosmic scales all the way down to tiny, subatomic ones. The scale of electrons, quarks, and gluons is the restrict to how far we’ve ever probed nature: all the way down to scales of ~10^-19 meters, the place these constructions stay point-like.
Unfortunately, this clarification fails fully. The mixture of quantum uncertainty and electrostatic repulsion completely can not account for the expertise of “strong matter” right here in our on a regular basis life.
It seems that that is associated to the query of why matter is steady in any respect. It needs to be a quantum mechanical purpose; it was recognized means again within the nineteenth century that no system made up of charged particles could be steady beneath the legal guidelines of classical electromagnetism alone. With the appearance of statistical mechanics, we got here to acknowledge that there’s a ground-state vitality, or a lowest-energy state, that any system of particles can possess. This is vital, as a result of any matter-based object can be a system of particles.
If these particles are electron-like, within the sense that they obey the statistics that electrons and particles prefer it do, that lowest-energy state is proportional to the variety of particles within the system. However, if these particles aren’t electron-like (fermions) and are as a substitute photon-like (bosons), the vitality of that state is far larger: proportional to not the variety of particles within the system however to that quantity raised to the 7/5 energy. (First proven by Freeman Dyson in 1967, and later rigorously confirmed by Joseph Conlon and Elliott Lieb.) The monumental worth of that vitality, which nonetheless follows the foundations of quantum uncertainty and electrostatic repulsion, teaches us that these two elements, alone, can not clarify the solidity or stability of matter.
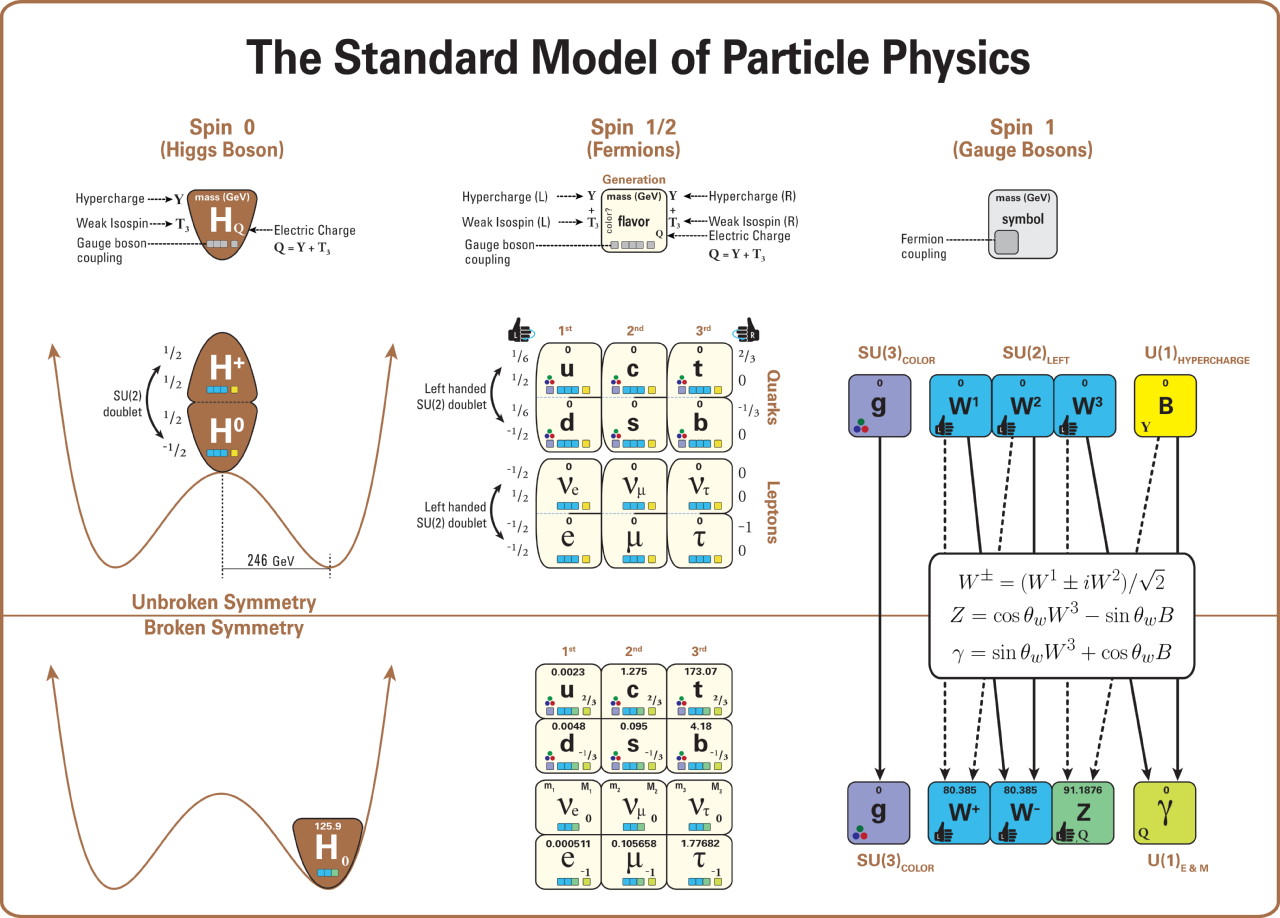
This diagram shows the construction of the usual mannequin (in a means that shows the important thing relationships and patterns extra fully, and fewer misleadingly, than within the extra acquainted picture based mostly on a 4×4 sq. of particles). In specific, this diagram depicts all the particles within the Standard Model (together with their letter names, lots, spins, handedness, expenses, and interactions with the gauge bosons: i.e., with the robust and electroweak forces). It additionally depicts the function of the Higgs boson, and the construction of electroweak symmetry breaking, indicating how the Higgs vacuum expectation worth breaks electroweak symmetry and the way the properties of the remaining particles change as a consequence. Neutrino lots stay unexplained.
Instead, we’re compelled to take a look at the distinction between these two courses of particles: fermions (named after Enrico Fermi) and bosons (named after Satyendra Bose). Bosons — particles just like the photon, gluon, and different particles with integer spin — comply with the maths you’re used to. You put one boson right into a system, and also you get one boson within the lowest-energy state. Put a second boson in there, and also you’ve bought two bosons within the lowest-energy state. Put a 3rd boson in there, and shortly there will probably be three bosons within the lowest vitality state. And so on, till you possibly can hear the Count’s voice from Sesame Street cackling, “Ah, ah, ah,” in your head.
Fermions, nevertheless, don’t. Particles with half-integer spin, like electrons, protons, and neutrons, obey a really completely different algorithm. Put one fermion right into a system, and it’ll drop to the lowest-energy state. But put a second fermion in there, and it may possibly solely drop to the lowest-energy state if it’s a quantum state that’s unoccupied by the earlier fermion(s) in there. The extra fermions you add, the extra “crowded” the system will get, which means that the one method to pile extra fermions in there may be to have them occupy progressively greater and better vitality ranges inside that system. This rule for fermions was decided by Wolfgang Pauli, and the precept that underlies it — the Pauli Exclusion Principle — is among the nice unavoidable details of our quantum Universe.
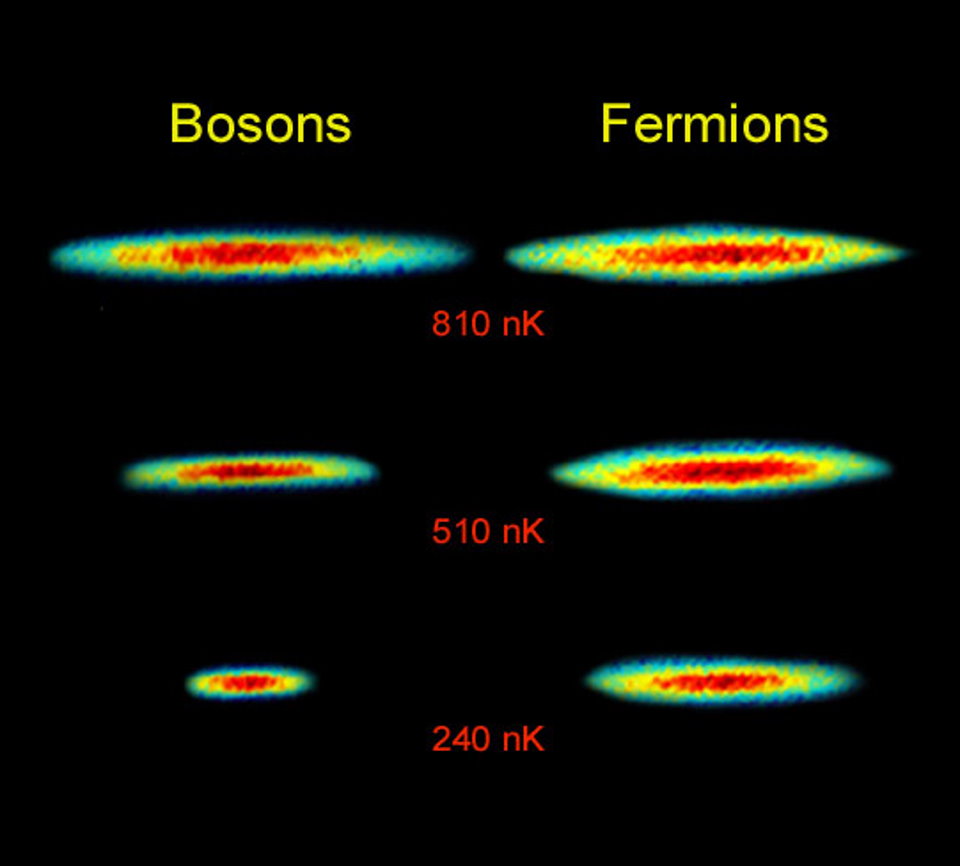
The Pauli exclusion precept prevents two fermions from coexisting in the identical quantum system with the identical quantum state. It solely applies to fermions, nevertheless, like quarks and leptons. It doesn’t apply to bosons, and therefore there isn’t any restrict to, say, the variety of equivalent photons that may coexist in the identical quantum state. It’s why fermion-containing stellar remnants, like white dwarfs and neutron stars, can maintain themselves up in opposition to gravitational collapse, because the Pauli Exclusion Principle limits the amount {that a} finite variety of fermions can occupy.
All of that’s advantageous and dandy for every particular person quantum system. But now, what occurs if you carry two programs, or two objects, which might be every made from atoms into contact with each other? Sure, there’s nonetheless quantum uncertainty and there’s nonetheless electrostatic repulsion, however now there’s a 3rd crucial issue at play: the Pauli exclusion rule that applies to electrons. When you push your thumb down onto the seat of your chair, the electrons in your thumb are already occupying all of the lowest-energy states which might be out there to them. Similarly, the electrons within the chair are already occupying all of the lowest-energy states out there to them.
This implies that the act of “pushing” your thumb into the chair is equal to making an attempt to push these occupied electrons into the identical quantum state as each other: to have their vitality ranges overlap. But these ranges are full; you possibly can’t match anymore electrons in there! The chair’s electrons must be bumped as much as larger energies with a view to transfer into (or by means of) your thumb, and your thumb’s electrons must be bumped as much as larger energies with a view to transfer into (or by means of) your chair. No matter how robust you’re, you merely don’t have sufficient power in your physique to beat the Pauli Exclusion Principle on this vogue.
And that, fairly merely, is the way you get the property of “impenetrability” that’s inherent to strong objects.

The vitality ranges and electron wavefunctions that correspond to completely different states inside a hydrogen atom, though the configurations are extraordinarily comparable for all atoms. The vitality ranges are quantized in multiples of Planck’s fixed, however the sizes of the orbitals and atoms are decided by the ground-state vitality and the electron’s mass. Only two electrons, one spin up and one spin down, can occupy every of those vitality ranges owing to the Pauli exclusion precept, whereas different electrons should occupy greater, extra voluminous orbitals. When you drop from a better vitality degree to a decrease one, you could change the kind of orbital you’re in in case you’re solely going to emit one photon, in any other case you’ll violate sure conservation legal guidelines that can not be damaged.
What occurs, then, in case you push as arduous as you probably can? It seems that it’s really simpler to interrupt bonds between atoms than it’s for atoms to move by means of each other, which means that you simply’re prone to both break your chair (or break your thumb) lengthy earlier than you overcome the Pauli Exclusion Principle. It’s this very purpose why, if you take a really sharp object, like a sharpened knife, it’ll “lower by means of” the bonds that bind atoms collectively, reasonably than merely move by means of the objects that they arrive into contact with. The electron could also be extraordinarily humble so far as particles go, however as a result of its standing as a fermion, it has the unbelievable property of forbidding all different equivalent fermions (i.e., different electrons) from occupying the quantum state that it occurs to be in.
This sort of stress, generally known as degeneracy stress, even retains the cores of planets, stars, and stellar remnants like white dwarfs and neutron stars from collapsing. Literally the one locations within the Universe the place the Pauli Exclusion Principle now not applies to things made out of atoms are black holes: the place there’s a lot matter condensed into such a small area of area that even gentle itself is forbidden from escaping from that area. That exact same precept prevents atoms, even if they’re principally empty area, from passing by means of one another, and in flip that provides matter its “solidity” that we’re so conversant in.

A white dwarf, a neutron star, or perhaps a unusual quark star are all nonetheless made from fermions. The Pauli degeneracy stress helps maintain up the stellar remnant in opposition to gravitational collapse, stopping a black gap from forming. Inside essentially the most large neutron stars, an unique type of matter, a quark-gluon plasma, is assumed to exist, with temperatures rising as much as ~1 trillion (10^12) Okay inside.
The Pauli Exclusion Principle doesn’t solely clarify why matter is strong, but in addition why it occupies the quantity of area that it does. Again: it isn’t simply the uncertainty precept and electrostatic repulsion that’s answerable for quantity; if matter had been made from bosons, it wouldn’t occupy area in the identical vogue that it does when it’s made from fermions. As I wrote earlier this yr:
“The hydrogen atom is small as a result of its electron is within the lowest-energy state allowable, the bottom state, and solely has one electron. Heavier atomic nuclei, nevertheless — like carbon, oxygen, phosphorus, or iron — have extra protons of their nuclei, requiring larger numbers of electrons inside them. If the lower-energy quantum states are all stuffed with electrons, then subsequent electrons should occupy higher-energy states, resulting in bigger electron orbits (on common) and “puffier” atoms that occupy larger volumes. […] The extra protons you’ve on the core of your atom, the extra electrons you’ve orbiting inside the outskirts of your atom. The extra electrons you’ve, the larger the variety of vitality states that have to be occupied. And the upper the vitality state of the highest-energy electrons inside your atom, the larger the quantity of bodily quantity your atom should occupy.”
As lengthy as matter is made up of fermions, it’s impenetrable to different objects which might be additionally made up of the identical sort of fermionic matter. To these of you who thought that the Pauli Exclusion Principle wasn’t such an enormous deal, keep in mind this: if it weren’t for that precept, and the fermionic nature of matter, one thing so simple as “sitting in a chair” could be a bodily impossibility!
The creator acknowledges Matt Strassler’s wonderful ebook, Waves in an Impossible Sea, for recounting the reason for, and story behind, the expertise of matter being strong and impenetrable.
Sign up for the Starts With a Bang e-newsletter
Travel the universe with Dr. Ethan Siegel as he solutions the largest questions of all